Non-Prescribers: Laboratory Staff
Non-prescribers are defined as staff/students who provide direct care to people, e.g. by dispensing and administering antimicrobials, monitoring treatment or caring for people with infection, but do not prescribe antimicrobials.
This includes:
- Staff/students from services related to antimicrobial stewardship (laboratory staff from microbiology or virology laboratories, pharmacy staff)
- All registered nursing and midwifery staff/students
- Other staff/students with many different roles and from different care settings, e.g. allied health professionals, dental nurses, clinical healthcare support workers, care home or home care support workers. However, not all statements will apply to all of these staff/students.
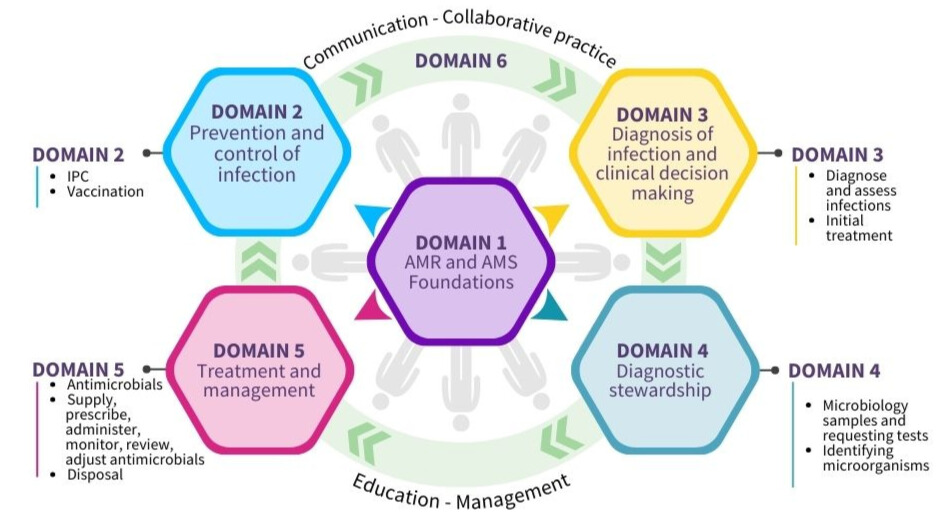
Please refer to the framework document for a full list of statements for all domains and staff groups.
Domain 1: Foundations - awareness of antimicrobial resistance and antimicrobial stewardship
Domain 2: Prevention and control of infection
Domain 3: Diagnosis of infection and clinical decision-making
Domain 4: Diagnostic stewardship
Domain 5: Treatment and management - appropriate use of antimicrobials
Domain 6: Communication, education, collaborative practice and management
Domain 1: Foundations - awareness of antimicrobial resistance and antimicrobial stewardship
Competency
Staff can demonstrate the following knowledge and awareness of Antimicrobial Resistance (AMR) and Antimicrobial Stewardship (AMS) and how this applies to their role and care setting.
| Antimicrobial Resistance |
|---|
|
1.1 Explain what antibiotics and other antimicrobials are and why they are important. |
|
1.2 Explain what AMR is and what causes it to develop. |
|
1.3 Describe how using antimicrobials incorrectly can cause AMR. |
|
1.4 Recognise that it is important not to use antimicrobials if they are not needed, especially those that affect a wide range of bacteria (broad spectrum). |
|
1.5 Recognise that the spread of microorganisms in the community and in hospitals can greatly increase infection rates, leading to the use of more antibiotics and higher AMR. |
| Impact of AMR |
|
1.6 Explain why AMR is a serious global health threat, because it can lead to more illness, deaths, and economic problems. |
|
1.7 Identify the positive and negative effects of using antimicrobials in humans. |
|
1.8 Recognise that AMR can harm individuals and also affect entire communities or populations. |
|
1.9 Describe how using antimicrobials incorrectly can harm the environment, including the damage caused by improperly disposing of drugs and the environmental benefits of switching from intravenous (IV) to oral antimicrobials. |
| Promoting prevention of AMR |
|
1.10 Encourage health and care workers, those in care, and the public to be aware of AMR and the proper use of antimicrobials. |
|
1.11 Highlight the importance of prevention and control of Healthcare Associated Infections (HAIs) and AMR. |
| Links between IPC, AMR and AMS and patient safety |
|
1.12 Explain how infection prevention and control (IPC), AMR, AMS, and the safety of people in care are connected. |
|
1.13 Raise awareness of the connection between AMS and IPC. |
| Antimicrobial stewardship |
|
1.14 Explain what AMS means and why it is important to protect antimicrobials. |
|
1.15 Recognise that using antimicrobials responsibly is essential to stop AMR from developing and spreading. |
|
1.16 Recognise that people with viral infections and bacterial infections that go away on their own (self-limiting infections) will not benefit from taking antibiotics. |
|
1.17 Explain the difference between broad-spectrum antibiotics, which target a wide range of bacteria, and narrow-spectrum antibiotics, which are designed to target specific bacteria, and when each type should be used. |
|
1.18 Recognise the importance of using antimicrobials wisely in both humans and animals to prevent the development of AMR. |
|
1.19 Identify ways to encourage the responsible use of antibiotics. |
|
1.20 Explain that using antimicrobials incorrectly can lead to treatment failure, make bacteria resistant to the drugs, and increase the risk of illness or complications. |
| Policies and guidance |
|
1.21 Find and access local and national policies and guidelines on AMR and AMS. |
|
1.22 Describe how they would use local and national policies on AMR and AMS in their role (Examples could be local antimicrobial guidelines or guidance from Scottish Antimicrobial Prescribing Group (SAPG) and the Scottish Intercollegiate Guidelines Network (SIGN)). |
| Roles and responsibilities |
|
1.23 Recognise their own role in AMS and consider any changes they can make in their practice to improve care and outcomes for those they care for and the public. |
|
1.24 Describe the roles, responsibilities, and skills of other health and social care workers – for example clinical nursing - involved in managing infections and AMS activities. |
|
1.25 Describe the roles and responsibilities of various members of AMS teams in managing and promoting responsible antimicrobial use. |
Domain 2: Prevention and control of infection
Competency
Staff can demonstrate an understanding of the importance of IPC and apply the principles in practice to reduce the spread of infection and AMR.
Infection prevention and control (IPC)
| SICPs and TBPs |
|---|
|
2.1 Explain what is meant by Standard Infection Control Precautions (SICPs) and Transmission Based Precautions (TBPs), and when to use them. |
|
2.2 Show how to use SICPs and TBPs in their health and/or social care working environments. |
| Role modelling and conduct |
|
2.3 Set a good example for other health and care workers and the public by following IPC principles and encouraging best practice. |
|
2.4 Explain how actions like getting the right vaccinations or staying home when sick help reduce the spread of infection and protect people they care for and other health and care workers. |
|
2.5 Recognise that health and social care staff have a duty to follow and encourage best practice for IPC. |
| Barriers and enablers |
|
2.6 Identify what makes good infection control easier or harder and know how to speak up if there is a problem. |
| Screening |
|
2.7 Explain why it is important to check for infections, (e.g. Methicillin-resistant Staphylococcus aureus (MRSA), when someone is admitted to hospital to help prevent the spread of infections. |
| Healthcare Built Environment |
|
2.8 Describe how the healthcare built environment affects IPC and the development of AMR. |
| IPC strategies |
|
2.9 Use methods and strategies to prevent and control HAIs, including infections from surgery, catheters, pneumonia, stomach flu, and others. |
|
2.10 Describe why it is important to have strategies to prevent infection in both community and healthcare settings, such as clean water, proper sanitation, waste management, and vaccinations. |
| IPC culture |
|
2.11 Develop and support the IPC culture in the workplace. |
| Challenging poor practice |
|
2.12 Recognise when infection control practices are not being followed properly and address it in the right way. |
| Healthcare and community associated infections |
|
2.13 Explain the difference between HAIs and community associated infections and their impact. |
| Alert organisms |
|
2.14 Explain what 'alert' organisms are and why they are important. These include: • Methicillin-resistant Staphylococcus aureus (MRSA) |
| Infectious diseases - causes |
|
2.15 Discuss the common sources or causes of infections in their area of work. |
| 2.16 Describe the potential for resistant organisms as a cause of infection in their place of work. |
Vaccination
| Role of vaccination |
|---|
| 2.17 Describe how vaccines help prevent infections and lower the risk of antimicrobial resistance (AMR). |
| Communication |
|
2.18 Identify strategies to boost vaccine confidence, such as providing clear, accurate information, addressing concerns and misconceptions, involving trusted healthcare professionals, and sharing positive testimonials from others who have been vaccinated. |
Domain 3: Diagnosis of infection and clinical decision-making
Competency
Staff can demonstrate an understanding of how infections are diagnosed and use this knowledge to support effective initial treatment as appropriate for their role.
Diagnosing and assessing infections
| Resistant microorganisms |
|---|
| 3.1 Explain what an antimicrobial resistant organism is. |
| Key factors |
| 3.2 Discuss factors that predispose to colonisation/infection with a resistant disease-causing organism, e.g. travel, recent hospitalisation or previous microbiology findings of resistant bacteria. |
| Diagnosing infections |
| 3.3 Describe what to consider when diagnosing and assessing infections. |
| Infections and colonisation |
| 3.6 Describe the differences between colonisation (e.g. isolation of bacteria from a venous leg ulcer with no signs of inflammation) and infection. |
| Infections and Inflammation |
| 3.7 Differentiate between inflammation and infection. |
| 3.8 Explain both infectious (bacterial and non-bacterial) and non-infectious (e.g. acute pancreatitis) causes of an inflammatory response. |
| Investigations and tools |
| 3.10 Use and interpret investigations that can help in informing diagnosis of an infection and in monitoring the response to treatment (e.g. microbiological investigations, biomarkers, point-of-care tests). |
Initial treatment
| Empirical treatment |
|---|
|
3.11 Explain the general principles of empirical antimicrobial therapy and pathogen-directed therapy (e.g. microbiology results). |
|
3.12 Describe how differences in microbial/antimicrobial susceptibility patterns impact on the choice of empirical therapy. |
| Empirical treatment – assessing severity |
|
3.14 Recognise the importance of initiating prompt effective empirical antimicrobial treatment in people with life-threatening infections (sepsis) following national guidance. |
Domain 4: Diagnostic stewardship
Competency
Staff can initiate, carry out and demonstrate an understanding of the role of appropriate microbiology sampling and testing to inform diagnosis and treatment of infections as appropriate for their role.
Microbiology samples and requesting tests
| Diagnostic stewardship |
|---|
|
4.1 Explain the principles and practice of diagnostic stewardship: Right test - right person - right time. |
|
4.2 Support the implementation of diagnostic stewardship in their own role. |
| Microbiology samples - general |
|
4.3 Describe their role in the identification, collection, handling, transportation and reporting of microbiological samples and test results. |
|
4.4 Explain the use of microbiological and other investigations to diagnose and monitor the response to treatment of infections and their complications and when they are not needed. |
|
4.5 Ensure the correct use and reporting of microbiological tests and diagnostic tools. |
| Taking microbiology samples |
| 4.6 Recognise the importance of adequate specimen collection or relevant tests before commencing treatment and during relevant stages of antimicrobial use (i.e. prior/during antibiotic treatment). |
|
4.7 Follow the correct procedures for choosing, collecting, storing, and transporting samples to the lab, according to the right policies and guidelines, and complete all necessary information, like clinical, demographic, and other relevant details, for each sample. |
Identifying microorganisms
| The role of the laboratory |
|---|
|
4.9 Describe how the microbiology laboratory helps to identify microorganisms and resistance patterns, supports case management, and provides information for IPC and AMS. |
| Reporting test results |
|
4.10 Make sure samples are handled quickly and within the required time frame. |
Domain 5: Treatment and management - appropriate use of antimicrobials
Competency
Staff can safely supply, prescribe, administer, monitor, review, adjust, and dispose of antimicrobials according to best practice guidelines as appropriate for their role.
Antimicrobials
| How do antimicrobials work - pharmacokinetics |
|---|
|
5.1 Explain the basic concepts of pharmacokinetics: route of therapy, concept of bioavailability, dosing frequency, therapeutic drug monitoring and clearance. |
| How do antimicrobials work - spectrum of activity |
|
5.2 Describe the major classes of antimicrobials, their mechanisms of action and their spectrum of antimicrobial activity in terms of Gram-positive, Gram-negative, anaerobic, and atypical bacteria and viruses, fungi and parasites. |
| Broad and narrow spectrum antimicrobials |
|
5.3 Describe broad-spectrum and narrow-spectrum antimicrobials and the contribution of broad-spectrum antimicrobials to AMR. |
|
5.4 Describe key features of specific infections, the first line choice narrow spectrum antibiotics to prescribe and length of antibiotic course in these scenarios (e.g. urinary tract infection (UTI), pneumonia, cellulitis). |
|
5.5 Identify clinical situations where broad-spectrum antimicrobials are warranted instead of narrow-spectrum. |
| Factors to consider when choosing an antimicrobial |
|
5.6 Explain that antimicrobials have different resistance potential (AWaRE categories of antibiotics from the WHO Essential Medicines List: Access – Watch - Reserve). |
| Preserving antimicrobials |
|
5.7 Recognise antimicrobials that should be preserved/reserved for treatment of specific infections e.g. carbapenem-producing organisms (CPO) or colistin-resistance or colistin resistant pathogens. |
Antimicrobial therapy
| Interpreting diagnostic test results |
|---|
|
5.13 Recognise laboratory results (i.e. culture and sensitivity) that demand flagging to healthcare workers and prompt intervention. |
|
5.14 Recognise factors that can affect diagnostic test results, e.g. someone is on antimicrobial therapy before the sample is taken. |
|
5.15 Interpret and use antimicrobial susceptibility testing results (in settings where they are commonly used) and other microbiology testing tools. |
|
5.16 Explain the principles of susceptibility reports. |
|
5.17 Interpret susceptibility reports. |
|
5.18 Explain how to interpret laboratory results/reports from the laboratory. |
| Antimicrobial therapy - general |
|
5.19 Appreciate the risk, benefits and limitations of the antimicrobial treatment in the context of the person treated and the care setting. |
|
5.20 Describe the difference between empiric therapy, targeted therapy using cultures and sensitivities and antimicrobial prophylaxis. |
|
5.21 Describe how antimicrobial selection would be different in empiric therapy, targeted therapy using cultures and sensitivities and antimicrobial prophylaxis. |
|
5.22 Describe the implications of commonly encountered resistance profiles in terms of management of infections (e.g. MRSA, VRE, ESBL, CPE). |
| Antimicrobial therapy - life threatening infections |
|
5.23 Describe why it is important to use local guidelines to initiate prompt, effective antimicrobial treatment in people with life-threatening infections. |
| Antimicrobial therapy - allergy |
|
5.29 Explain why it is essential that an accurate diagnosis of an allergy to an antimicrobial is based on history and/or laboratory tests. |
|
5.32 Explain why accurately documenting a person’s allergy to an antimicrobial and allergy de-labelling is important. |
| Using 5 r's - Right drug, dose, time, route, person |
|
5.33 Discuss the importance of antimicrobial choice, dosage, frequency, duration, preparation and administration of antimicrobials. |
| Inappropriate prescribing |
|
5.38 Recognise the immediate and long-term consequences of inappropriate antimicrobial prescription on the individual and the environment. |
| Administering antimicrobials |
|
5.55 Explain the different methods of the administration of antimicrobials. |
Supply and disposal
| Disposing antimicrobials |
|---|
|
5.76 Dispose of unused antimicrobial medicines safely. |
Domain 6: Communication, education, collaborative practice and management
Competency
Staff can effectively collaborate and communicate with co-workers, the wider team and those they care for and their families/carers, provide educational support on the use of antimicrobials and promote stewardship activities as appropriate for their role.
Communication and education
| Communication |
|---|
|
6.1 Recognise the importance of communicating with people they care for and other staff. |
| Communication with clinicians |
|
6.5 Maintain effective communication and work together with doctors, antimicrobial management teams, IPC professionals, hospital epidemiologists, and other health and care workers involved in AMS activities. |
|
6.6 Explain why it is important to work with experts, like infection specialists, when their help is needed. |
|
6.7 Communicate the risks of the development and transmission of AMR within and outside of multidisciplinary teams. |
|
6.8 Optimise clinical collaboration through advice on laboratory tests and timely communication of results. |
| Education on safe and appropriate use of antimicrobials |
|
6.17 Encourage ways to help individuals or communities learn how to use antimicrobials correctly. |
| Education - when antimicrobials are not needed |
|
6.20 Educate those they care for and their carers, and other supporting clinical staff about: • self-care (based on evidence, for example with pain relief, rest, and fluids) |
| Education - identify learning needs |
| 6.22 Identify their own learning needs and goals for development in AMS. |
Collaborative practice and management
| Team working |
|---|
|
6.23 Get involved in AMS as part of a team with members from different areas of expertise. |
|
6.24 Explain why it is important for healthcare professionals involved in giving antimicrobials (like prescribing, delivering, and supplying) to all understand the same antimicrobial treatment policy decisions, the quantity/quality of antimicrobial use, and what results are expected. |
|
6.25 Ask for help and advice from the AMS team when necessary. |
|
6.26 Work with the AMS team to help ensure antibiotics are used in the best way. |
| Quality Improvement |
|
6.28 Engage in national AMS initiatives aimed at supporting national policy and quality improvement, e.g. IV to oral switch therapy (IVOST). |
|
6.29 Explain and engage with any locally or nationally agreed quality measures (and metrics as appropriate) for assessing antimicrobial prescriptions (e.g. compliance with guidance, adverse events, complications, reviews of antibiotic therapy at 48-72 hours in hospitalised patients). |
| Prioritisation of AMS |
|
6.30 Support public health campaigns about AMR, for example:
|
| Previous page 04 - About the framework |
Next page 06 - Education to support the framework |
